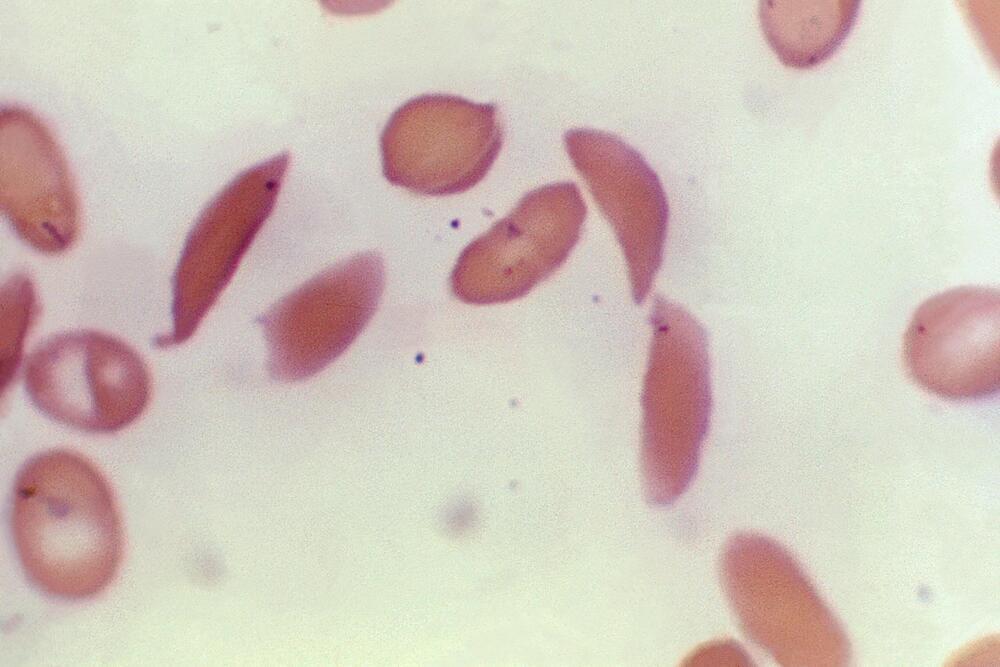
The Food and Drug Administration approved the use of Casgevy, a CRISPR gene-editing therapy, for treating the serious blood disorder transfusion-dependent beta thalassemia—the second major approval for the emerging therapy.
FDA Approves New CRISPR Gene-Editing Treatment
Posted in biotech/medical, genetics