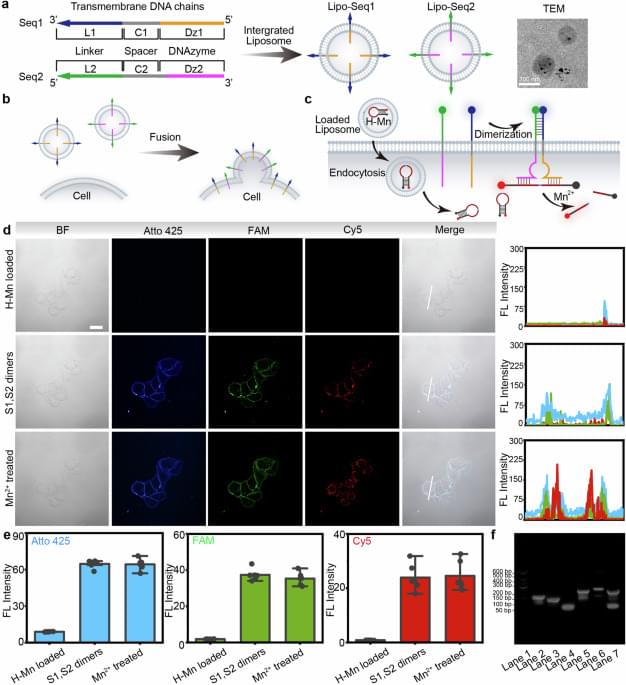
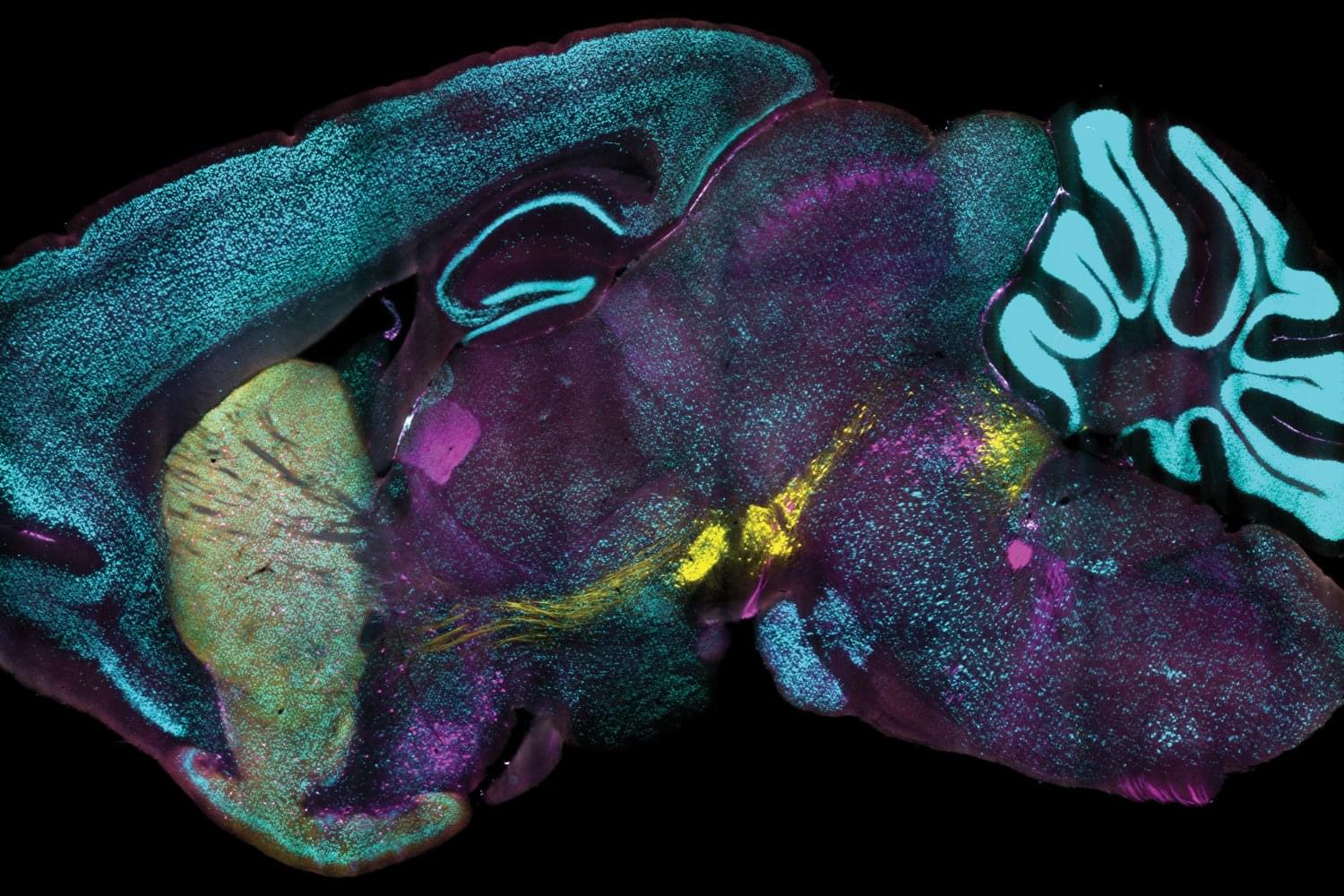
A new technology developed at MIT enables scientists to label proteins across millions of individual cells in fully intact 3D tissues with unprecedented speed, uniformity, and versatility. Using the technology, the team was able to richly label large tissue samples in a single day. In their new study in Nature Biotechnology, they also demonstrate that the ability to label proteins with antibodies at the single-cell level across large tissue samples can reveal insights left hidden by other widely used labeling methods.
Profiling the proteins that cells are making is a staple of studies in biology, neuroscience, and related fields because the proteins a cell is expressing at a given moment can reflect the functions the cell is trying to perform or its response to its circumstances, such as disease or treatment. As much as microscopy and labeling technologies have advanced, enabling innumerable discoveries, scientists have still lacked a reliable and practical way of tracking protein expression at the level of millions of densely packed individual cells in whole, 3D intact tissues. Often confined to thin tissue sections under slides, scientists therefore haven’t had tools to thoroughly appreciate cellular protein expression in the whole, connected systems in which it occurs.
“Conventionally, investigating the molecules within cells requires dissociating tissue into single cells or slicing it into thin sections, as light and chemicals required for analysis cannot penetrate deep into tissues. Our lab developed technologies such as CLARITY and SHIELD, which enable investigation of whole organs by rendering them transparent, but we now needed a way to chemically label whole organs to gain useful scientific insights,” says study senior author Kwanghun Chung, associate professor in The Picower Institute for Learning and Memory, the departments of Chemical Engineering and Brain and Cognitive Sciences, and the Institute for Medical Engineering and Science at MIT. “If cells within a tissue are not uniformly processed, they cannot be quantitatively compared. In conventional protein labeling, it can take weeks for these molecules to diffuse into intact organs, making uniform chemical processing of organ-scale tissues virtually impossible and extremely slow.”