The implications of early telencephalic development in cortical disorders remain elusive. Here, the authors define risk gene dynamics and perturbation effects in neural stem cells, revealing vulnerability phases during early human corticogenesis.



They look like futuristic eyewear. But the goggles developed by Samuel Achilefu, PhD, and his team at the School of Medicine have a much greater purpose: They help surgeons see and remove cancer. Achilefu discusses his journey from childhood to the development of the goggles, to what he hopes is yet to come.

Copper is an essential trace element for normal development and function throughout the body, including the central nervous system (CNS). Alterations to cellular copper levels result in severe neurological consequences and are linked to a range of CNS disorders, positioning treatments that restore copper balance as promising therapies for these disorders. However, despite the clear relationship between copper balance and CNS health, there are limited tools to measure copper levels in vivo in humans. This constitutes a significant challenge for both diagnosing disorders of copper imbalance and monitoring the efficacy of copper-altering treatments for these disorders. Here we report the synthesis and characterization of Fluorine-labeled Naphthalimide Copper sensor 1 (F-NpCu1), a fluorescent sensor for copper that contains a fluorine atom for future radiolabeling for clinical application. We demonstrate that the probe exhibits good stability and is highly selective for copper above other transition metals present in biological tissues. Copper binding promotes covalent bond formation between the sensor and proximal cellular proteins. F-NpCu1 is nontoxic and can be measured using fluorescence microscopy in living cells and fixed tissue sections from both mouse brain and pancreas. Furthermore, F-NpCu1 exhibits good blood-brain-barrier permeability and can report differences in brain copper levels induced by copper modulating therapies in living mice using intravital fluorescence microscopy. This study represents a promising advance toward the development of the first clinical tool for measuring copper in living humans, including in the CNS, with radiolabeling studies underway to develop 18F-NpCu1 for PET imaging of copper in vivo.
This is a sci-fi documentary looking at the future of genetic engineering and how it applies to space exploration, astronauts, terraforming planets and even Earth.
What is DNA, and how can it be engineered. What is CRISPR, and the future technology used in genetic engineering and biotechnology.
Personal inspiration in creating this video came from: Jurassic Park (the book), and The Expanse TV show (the protomolecule).
Other topics in the video include: how genetic engineering can change food allergies, cryosleep astronauts using hibernation biology borrowed from bears, squirrels and hedgehogs, engineering plants for terraforming other planets, and entries from The Encyclopedia of the Future.
PATREON
The third volume of ‘The Encyclopedia of the Future’ is now available on my Patreon.
Visit my Patreon here: https://www.patreon.com/venturecity.

Researchers from Columbia Engineering have established a framework for the design of bioactive injectable hydrogels formulated with extracellular vesicles (EVs) for tissue engineering and regenerative medicine applications.
Published in Matter, Santiago Correa, assistant professor of biomedical engineering at Columbia Engineering, and his collaborators describe an injectable hydrogel platform that uses EVs from milk to address longstanding barriers in the development of biomaterials for regenerative medicine.
EVs are particles naturally secreted by cells and carry hundreds of biological signals, like proteins and genetic material, enabling sophisticated cellular communication that synthetic materials cannot easily replicate.

Researchers at UT Southwestern Medical Center have discovered how a hormone interacts with a receptor on the surface of immune cells to shield cancer cells from the body’s natural defenses.
The findings, published in Nature Immunology, could lead to new immunotherapy approaches for treating cancer as well as potential treatments for inflammatory disorders and neurologic diseases.
“Myeloid cells are among the first group of immune cells recruited to tumors, but very quickly these tumor-fighting cells turn into tumor-supporting cells. Our study suggests that receptors on these myeloid cells get stimulated by this hormone and end up suppressing the immune system,” said Cheng Cheng “Alec” Zhang, Ph.D., Professor of Physiology and a member of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern. Dr. Zhang co-led the study with first author Xing Yang, Ph.D., a postdoctoral researcher in the Zhang Lab.

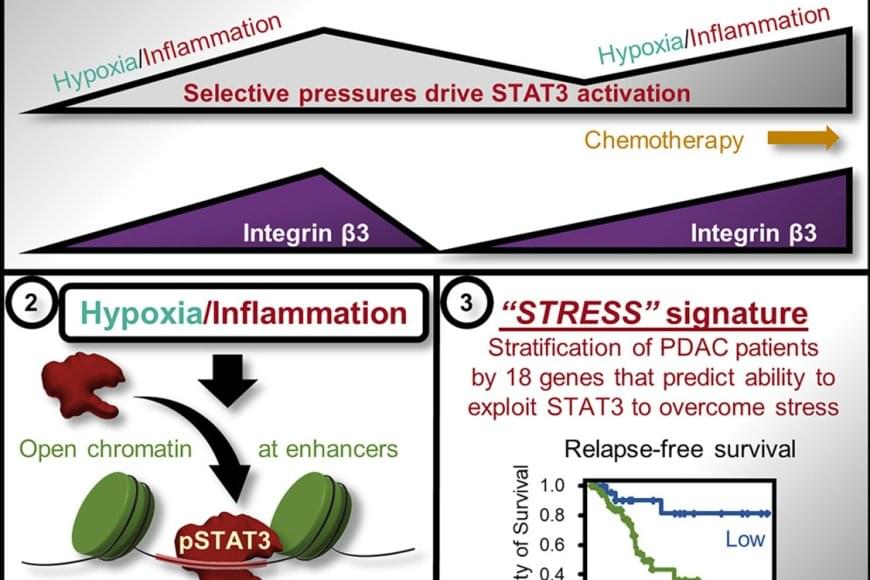
Precancerous cells must adapt to and overcome cellular stress and inflammation in order to progress and form malignant tumors. Now, researchers have identified a link between stress and inflammation and pancreatic ductal adenocarcinoma (PDAC), one of the most aggressive and lethal types of cancer. The findings could serve as an early warning system for the disease, leading to the detection of PDAC before it becomes life-threatening.
Previous studies have shown that inflammation and cellular stress activate a protein called STAT3 — short for signal transducer and activator of transcription 3 — in pancreas cells, promoting tumor initiation, adaptation to stress and resistance to treatment. How STAT3 accomplishes this has not been understood until now.
In the current study, the researchers discovered that in some cancer cells, STAT3 is able to activate specific genes critical for adaptation to stress and inflammation. They found: