NOTE FROM TED: Please do not look to this talk for medical advice. We’ve flagged this talk, which was filmed at a TEDx event, because it appears to fall outside TEDx’s content guidelines. Gene therapy remains an emerging field of study. The speaker’s claims only reflect her personal views which are not corroborated by scientific evidence. TEDx events are independently organized by volunteers. The guidelines we give organizers are described in more detail here: http://storage.ted.com/tedx/manuals/tedx_content_guidelines.pdf
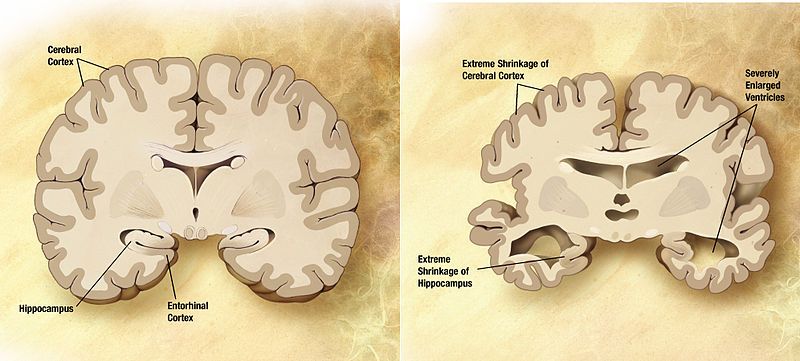
Liz Parrish is the CEO of BioViva USA Inc, a company committed to increasing health span and combatting the biggest diseases of our time, the diseases of aging. Liz’s talk introduces us to our species’ age-long battle with disease, our successes and achievements, and our new modern enemies to ultimate health. By using the evidence of what science has achieved and what model organisms can tell us about our journey forward, Liz shows us that with the right mentality we can defeat our foes and obtain the “golden sceptre of health”. With the current advances in gene therapy, we are on the brink of living very different lives with an unlimited amount of genetic choices. Did you know that your age is the risk factor in mortality? Listen to this talk and prepare yourself for the future.
This talk was given at a TEDx event using the TED conference format but independently organized by a local community.