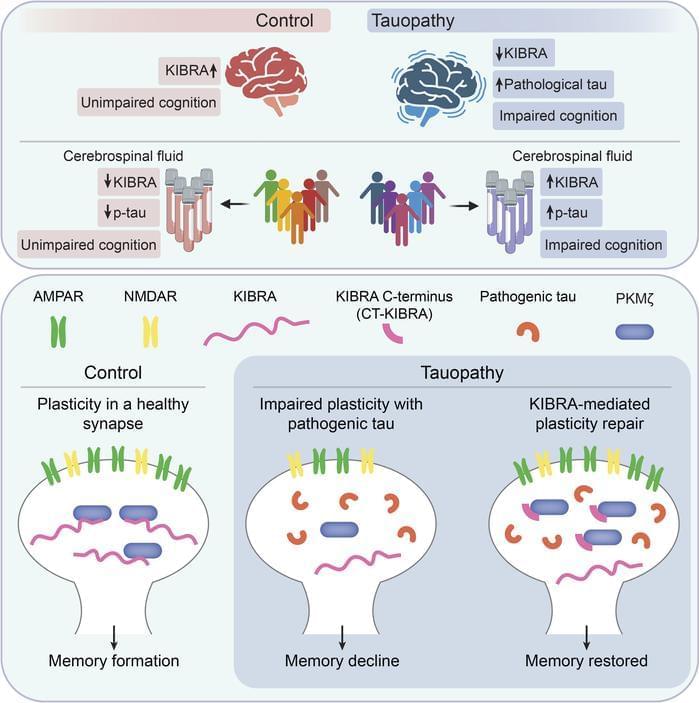
Representing a key mechanism that underlies memory loss in Alzheimer’s disease (AD) and related tauopathies. Here, we found that reduced levels of the memory-associated protein KIdney/BRAin (KIBRA) in the brain and increased KIBRA protein levels in cerebrospinal fluid are associated with cognitive impairment and pathological tau levels in disease. We next defined a mechanism for plasticity repair in vulnerable neurons using the C-terminus of the KIBRA protein (CT-KIBRA). We showed that CT-KIBRA restored plasticity and memory in transgenic mice expressing pathogenic human tau; however, CT-KIBRA did not alter tau levels or prevent tau-induced synapse loss. Instead, we found that CT-KIBRA stabilized the protein kinase Mζ (PKMζ) to maintain synaptic plasticity and memory despite tau-mediated pathogenesis. Thus, our results distinguished KIBRA both as a biomarker of synapse dysfunction and as the foundation for a synapse repair mechanism to reverse cognitive impairment in tauopathy.
1Buck Institute for Research on Aging, Novato, California, USA.
2Memory and Aging Center, Department of Neurology, University of California San Francisco, San Francisco, California, USA.
3Gladstone Institutes, San Francisco, Califoria, USA.
4Weill Institute for Neurosciences, Department of Pathology, University of California San Francisco, San Francisco, California, USA.