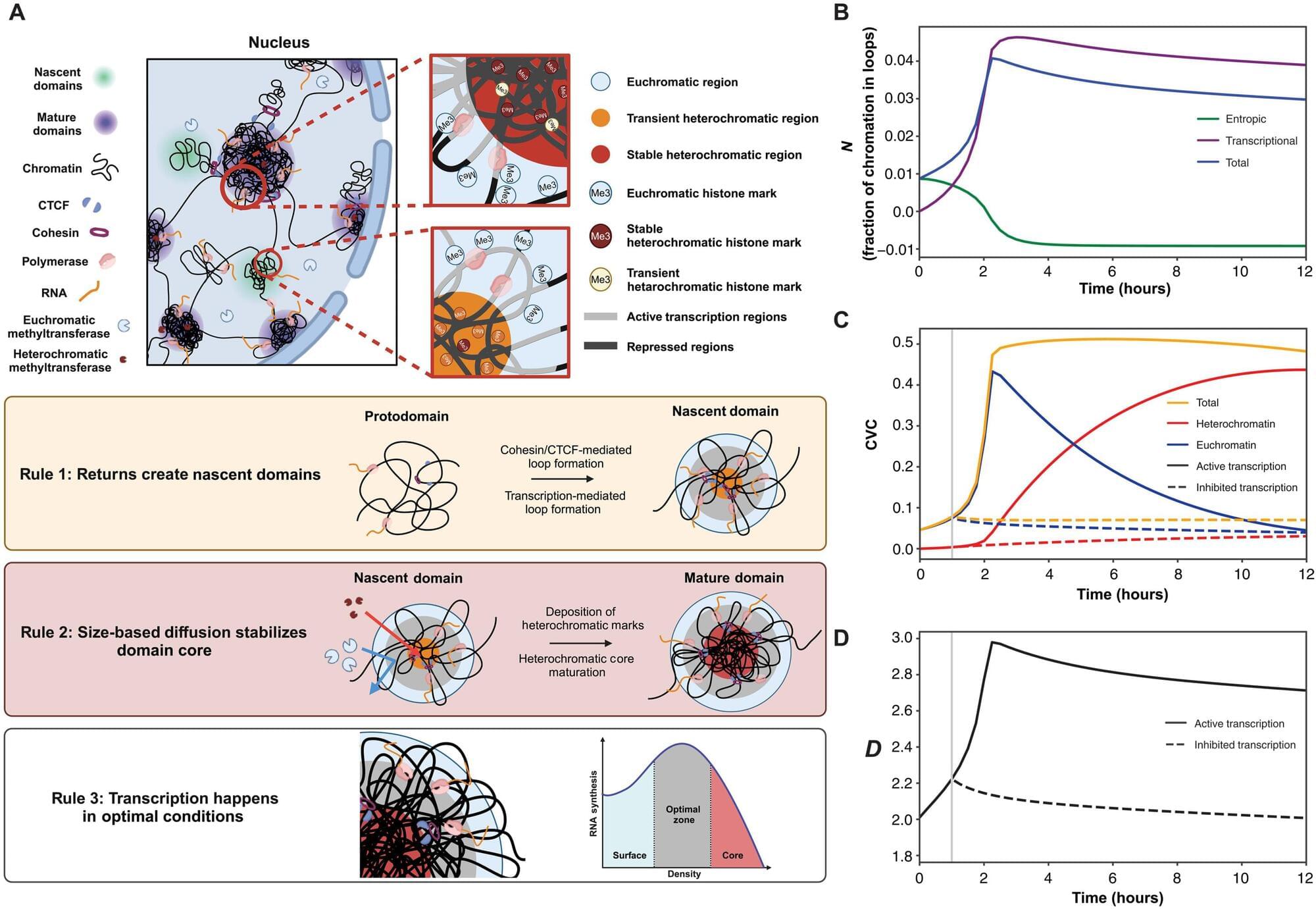
Northwestern Medicine scientists have discovered new details about how the human genome produces instructions for creating proteins and cells, the building blocks of life, according to a pioneering new study published in Science Advances.
While it’s understood that genes function as a set of instructions for creating RNA, and thus proteins and cells, the fundamental process by which this occurs has not been well-studied due to technological limitations, said Vadim Backman, Ph.D., the Sachs Family Professor of Biomedical Engineering and Medicine, who was senior author of the study.
“It is still not fully understood how, despite having the same set of genes, cells turn into neurons, bones, skin, heart, or roughly 200 other kinds of cells, and then exhibit stable cellular behavior over a human lifespan which can last for more than a century—or why aging degrades this process,” said Backman, who directs the Center for Physical Genomics and Engineering at Northwestern. “This has been a long-standing open question in biology.”