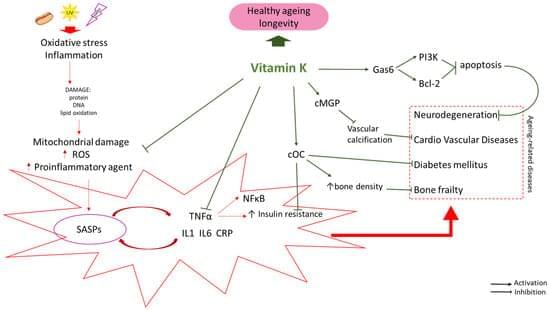
This conference will explore the interdisciplinary interrelations of science, technology and society in addressing the challenges of population aging. By bringing together leading voices in the longevity space and public figures, the Longevity Nation conference will strongly contribute to increasing the synergy of science, technology and aging society, and help advance ethical scientific and technological solutions for healthy longevity for the benefit of the entire society. Building on Israel’s strengths in this area, this conference will help build the supportive longevity ecosystem in Israel, boost the prominence of the field in Israel and enhance Israel’s international standing and cooperation in the Longevity Field. It will help build up longevity R&D and Education support programs, for stakeholders in Israel and international collaborators.