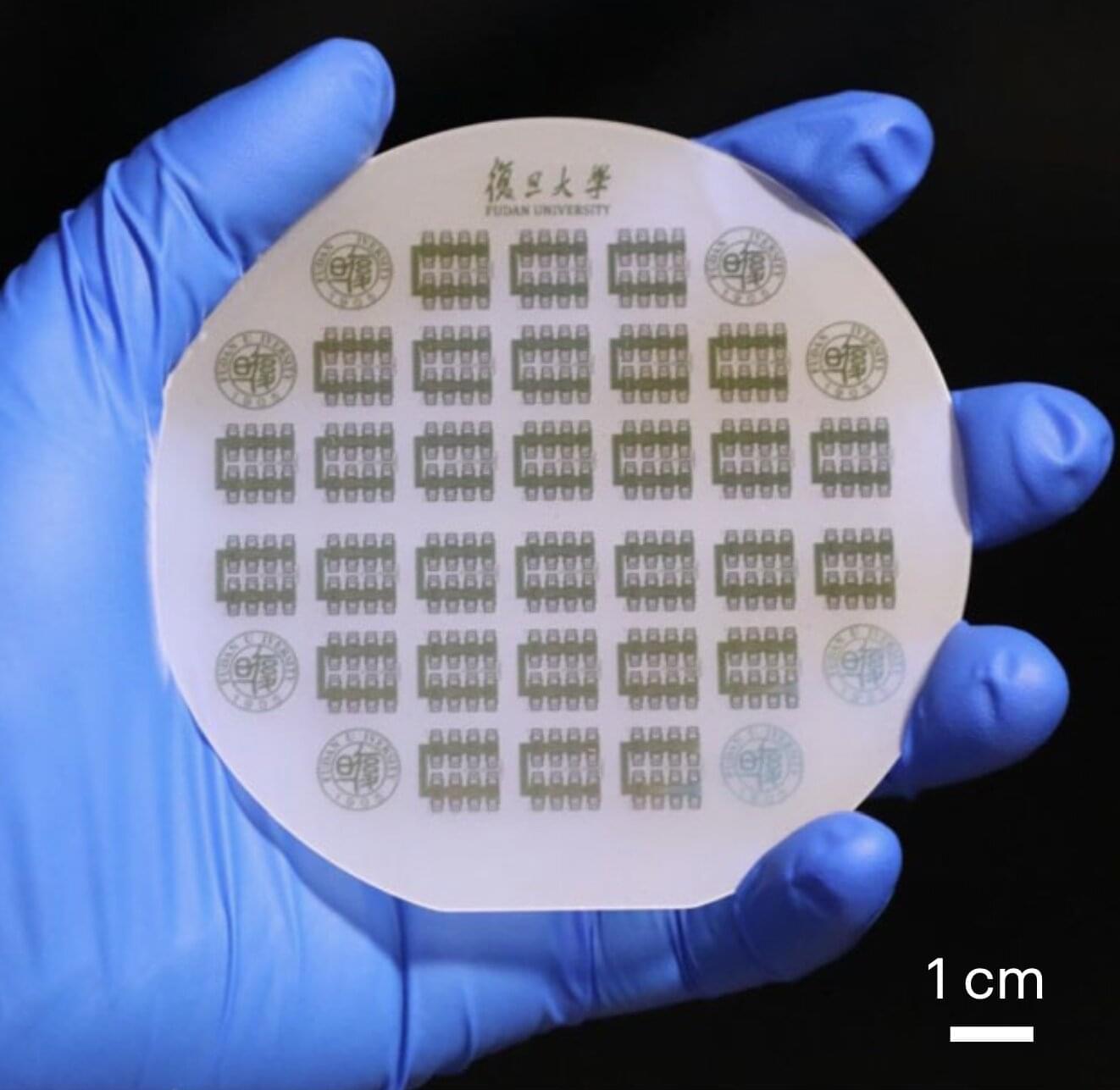
To further advance wireless communication systems, electronics engineers have been trying to develop new electronic circuits that operate in the microwave frequency range (1–300 GHz), while also losing little energy while transmitting signals. Ideally, these circuits should also be more compact than existing solutions, as this would help to reduce the overall size of communication systems.
Most of the microwaves integrated in current communication systems are made of bulk materials, such as silicon or gallium arsenide. While these circuits have achieved good results so far, both their size and power consumption have proved to be difficult to reduce further.
Two-dimensional (2D) semiconducting materials, which are made up of a single atomic layer, could overcome the limitations of bulk materials, as they are both thinner and exhibit advantageous electrical properties. Among these materials, molybdenum disulfide (MoS₂), has been found to be particularly promising for the development of microwave circuits and other components for communication systems.