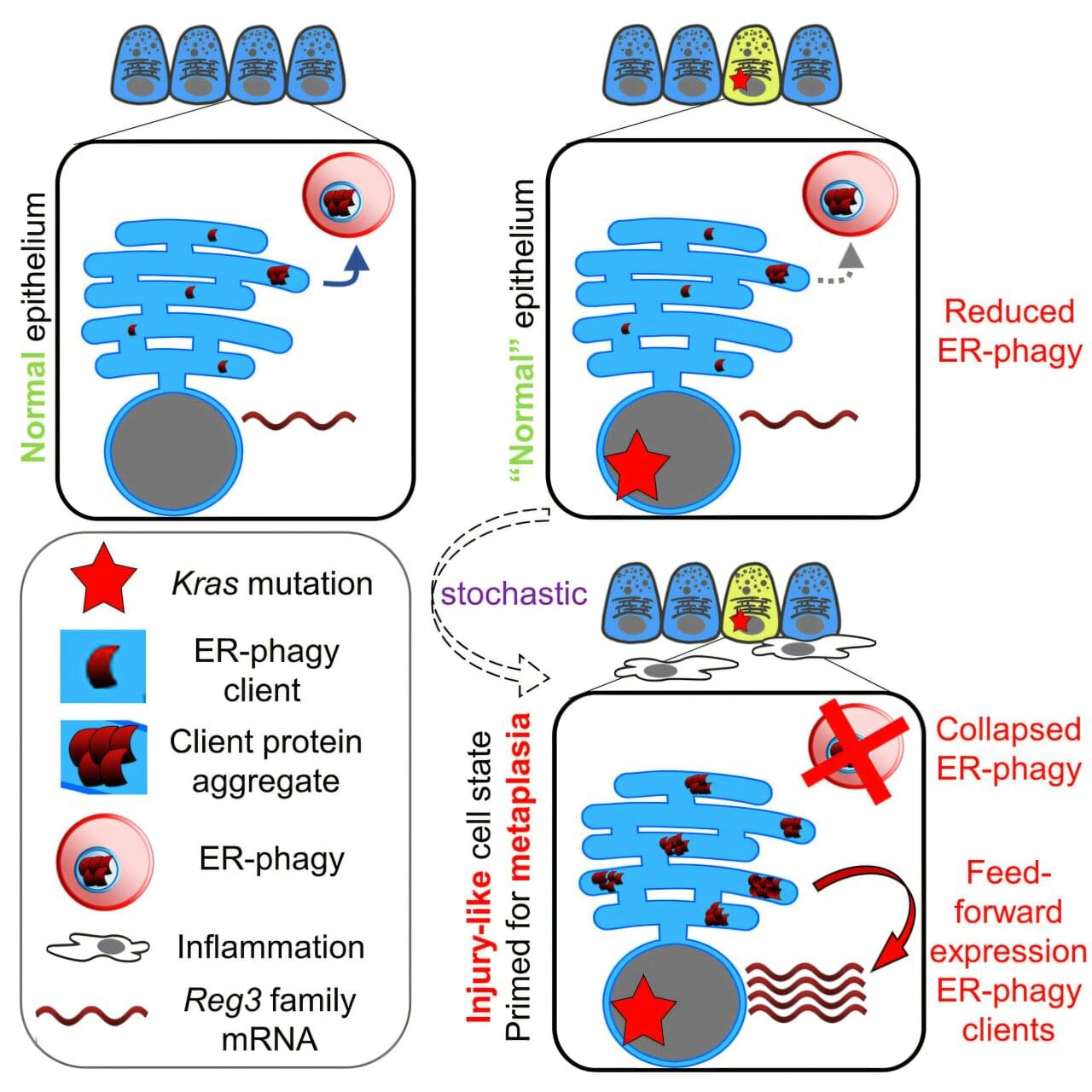
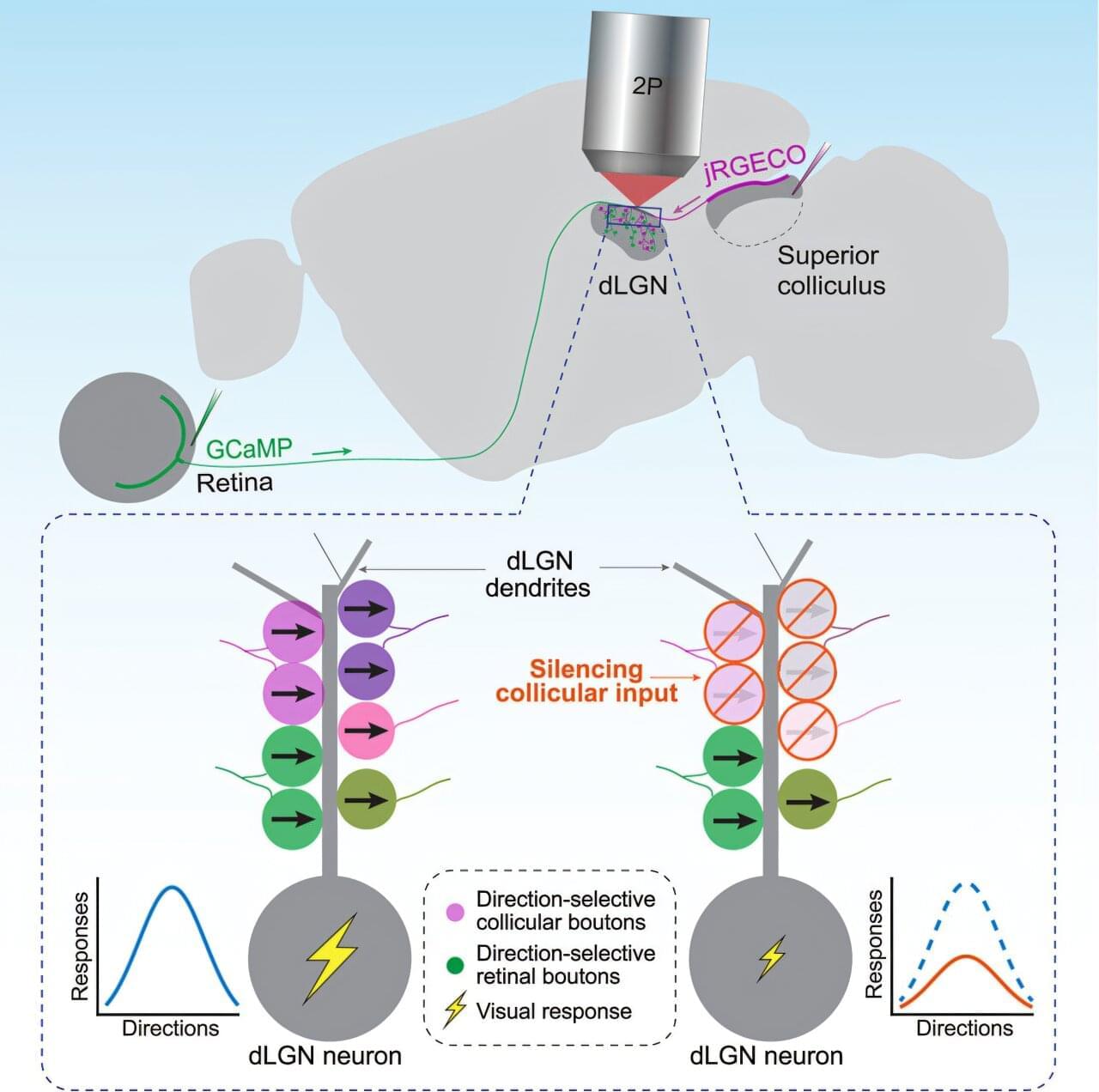
For centuries, philosophers and scientists have speculated about the mysterious connections between human minds. Now, new research in neuroscience suggests that our brains may be linked in a very real, physical way — through extremely low frequency (ELF) electromagnetic waves.
Neuroscientists studying brain activity have found that the human brain not only generates its own electrical signals but also emits faint electromagnetic waves at extremely low frequencies. These ELF waves, which typically range from 1 to 30 Hertz, are so subtle that they were once thought to be biologically insignificant. However, recent experiments show that these signals can extend beyond the skull and interact with the electromagnetic environment around us.
What’s even more remarkable is that similar ELF patterns appear across different individuals, raising the possibility that brains can “tune in” to each other through this shared frequency spectrum.