There are Sirt6 activators on the market, but since we are not seeing any major news about results I would question their value.
SPONSOR: Longevity. Technology — https://www.longevity.technology/?utm_source=SSS&utm_medium=…aign=Sirt6
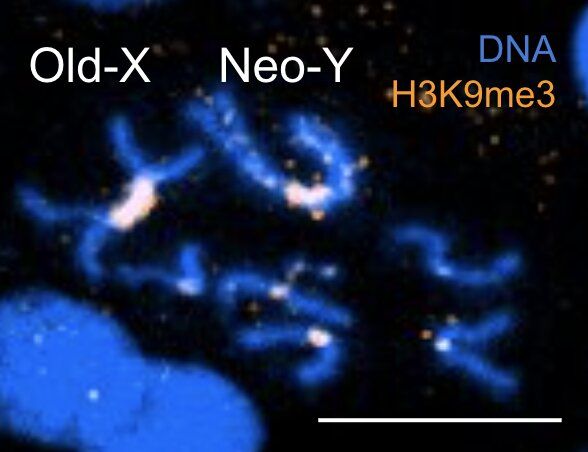
Sirtuins are highly conserved proteins that are involved in a variety of important cellular processes such as DNA repair, metabolism and circadian rhythms. The mammalian sirtuins (SIRT1-7) are a family of proteins that carry out NAD+-dependent protein deacylation and mono-ADP-ribosylation. These modifications on proteins can influence their stability, localisation within a cell and activity.
In the late 90s interest in sirtuins bloomed as it was found yeast lived 30% longer when they had an additional copy of a yeast sirtuin, Sir2. Similar studies have now been performed in mice, but whilst overexpression of SIRT1 in mice does not result in lifespan extension, overexpression of SIRT6 does. This has led to SIRT6 being referred to as the longevity sirtuin. However, there seems to be some sex-and mouse strain-dependent differences. So, in the remainder of the video, we will discuss what you need to know about SIRT6 including it’s proposed cellular activities, it’s association with longevity and how SIRT6 activation using allosteric activators could have future therapeutic potential.
TIMESTAMPS: