Finnish study suggests antibiotics can be an effective alternative to going under the knife



DNA methylation plays a critical role in gene expression regulation and has emerged as a robust biomarker of biological age. This modification will become heavier or site drift along with aging. Recently, it is termed epigenetic clocks—such as Horvath, Hannum, PhenoAge, and GrimAge—leverage specific methylation patterns to accurately predict age-related decline, disease risk, and mortality. These tools are now widely applied across diverse tissues, populations, and disease contexts. Beyond age-related loss of methylation control, accelerated DNA methylation age has been linked to environmental exposures, lifestyle factors, and chronic diseases, further reinforcing its value as a dynamic and clinically relevant marker of biological aging. DNA methylation is reshaping our understanding of aging and disease risk, with promising implications for preventive medicine and interventions aimed at promoting healthy longevity. However, it must be admitted that some challenges remain, including limited generalizability across populations, an unclear mechanism, and inconsistent longitudinal performance. In this review, we examine the biological foundations of DNA methylation, major advances in epigenetic clock development, and their expanding applications in aging research, disease prediction and health monitoring.
Aging is a complex, multifactorial process that affects nearly all biological systems. While chronological age simply measures the passage of time from birth, biological age reflects the functional state and health of an individual’s tissues and organs (Kiselev et al., 2025). This distinction is critical, as individuals of the same chronological age often exhibit markedly different biological conditions, disease risks, and mortality trajectories (Dugue et al., 2018). Therefore, biological age potentially serves as a more meaningful measure of aging-related decline and is increasingly used to assess overall health status, predict disease onset, and evaluate the effectiveness of interventions aimed at promoting healthy longevity (Dugue et al., 2018; Petkovich et al., 2017).
Among various biomarkers proposed to estimate biological age, epigenetic modifications—particularly DNA methylation—have emerged as one of the most reliable and informative (Dugue et al., 2018). In epigenetics, DNA methylation involves the addition of a methyl group to the 5′ position of cytosine residues, typically at CpG dinucleotides, which can regulate gene expression without altering the underlying DNA sequence. Moreover, DNA methylation can be accurately measured by sequencing at methylated sites with bisulfate treatment (Zhang et al., 2012). Age-related changes in DNA methylation pattern are not random; they occur at specific genomic locations. These methylated sites are picked and constitute come patterns, by which scientists can construct “epigenetic clocks” to precisely estimate a person’s biological age based on their DNA modification. As people grow older, their methylation profiles shift in predictable ways (Kiselev et al., 2025; Horvath, 2013; Horvath and Raj, 2018).


A team led by researchers at Chalmers University of Technology, Sweden, has succeeded in identifying biomarkers for Parkinson’s disease in its earliest stages, before extensive brain damage has occurred. The biological processes leave measurable traces in the blood, but only for a limited period.
The discovery thus reveals a window of opportunity that could be crucial for future treatment, but also for early diagnosis via blood tests, which could begin to be tested in health care within five years.
Parkinson’s is an endemic disease with over 10 million people affected globally. As the world’s population grows older, this number is expected to more than double by 2050. At present, there is neither an effective cure nor an established screening method for detecting this chronic neurological disorder at an early stage before it has caused significant damage to the brain.

Scientists continue to mine data gathered by NASA’s Kepler Space Telescope, retired in 2018, and continue to turn up surprises. A new paper reveals the latest: a possible rocky planet slightly larger than Earth, orbiting a sun-like star about 146 light-years away. The candidate planet, HD 137010b, might be remarkably similar to Earth, but it has one potentially big difference: It could be colder than perpetually frozen Mars.
A promising Earth-sized exoplanet emerges An international science team published a paper on the discovery, “A Cool Earth-sized Planet Candidate Transiting a Tenth Magnitude K-dwarf From K2,” in The Astrophysical Journal Letters on Jan. 27, 2026. The team was led by astrophysics Ph.D. student Alexander Venner of the University of Southern Queensland, Toowoomba, Australia, now a postdoctoral researcher at the Max Planck Institute for Astronomy, Heidelberg, Germany.
The orbital period of the planet—listed as a “candidate” pending further confirmation—is likely to be similar to Earth’s, around one year. Planet HD 137,010 b also might fall just within the outer edge of its star’s “habitable zone,” the orbital distance that could allow liquid water to form on the planet’s surface under a suitable atmosphere.

#Quantum #CyberSecurity
Quantum computing is not merely a frontier of innovation; it is a countdown. Q-Day is the pivotal moment when scalable quantum computers undermine the cryptographic underpinnings of our digital realm. It is approaching more rapidly than many comprehend.
For corporations and governmental entities reliant on outdated encryption methods, Q-Day will not herald a smooth transition; it may signify a digital catastrophe.
Comprehending Q-Day: The Quantum Reckoning
Q-Day arrives when quantum machines using Shor’s algorithm can dismantle public-key encryption within minutes—a task that classical supercomputers would require billions of years to accomplish.

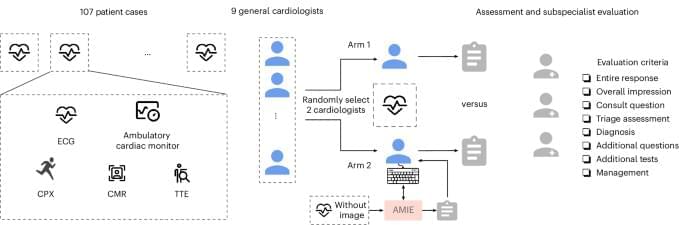
In a randomized study involving 9 general cardiologists and 107 real-world patient cases, assistance from a specifically tailored large language model resulted in preferable responses on complex case management compared to physicians alone, as rated by specialist cardiologists using a multidimensional scoring rubric.

The human body is often described in parts—different limbs, systems, and organs—rather than something fully interconnected and whole. Yet many bodily processes interact in ways we may not always recognize. For example, researchers at the University of Missouri School of Medicine may have found a link between high blood pressure and an overactive nervous system.
The paper is published in the journal Cardiovascular Research.
High blood pressure, also called hypertension, is a common cardiovascular condition and a risk factor for multiple diseases and sudden health concerns like stroke or heart attack.

Two or more graphene layers that are stacked with a small twist angle in relation to each other form a so-called moiré lattice. This characteristic pattern influences the movement of electrons inside materials, which can give rise to strongly correlated states, such as superconductivity.
Researchers at Ecole Polytechnique Fédérale de Lausanne, Freie Universität Berlin and other institutes recently uncovered a strong superconductivity in a supermoiré lattice, a twisted trilayer graphene structure with broken symmetry in which several moiré patterns overlap. Their paper, published in Nature Physics, could open new possibilities for the design of quantum materials for various applications.
“Fabricating a twisted trilayer graphene device with two distinct twist angles was not our original intention,” Mitali Banerjee, senior author of the paper, told Phys.org. “Instead, we aimed to make a device in which the two twist angles are identical in magnitude (magic-angle twisted trilayer). During our measurements, however, my student Zekang Zhou found that the phase diagram of this device differs fundamentally from that of magic-angle twisted trilayer graphene.”