Lithium-ion batteries have a lot of advantages. They charge quickly, have a high energy density, and can be repeatedly charged and discharged.
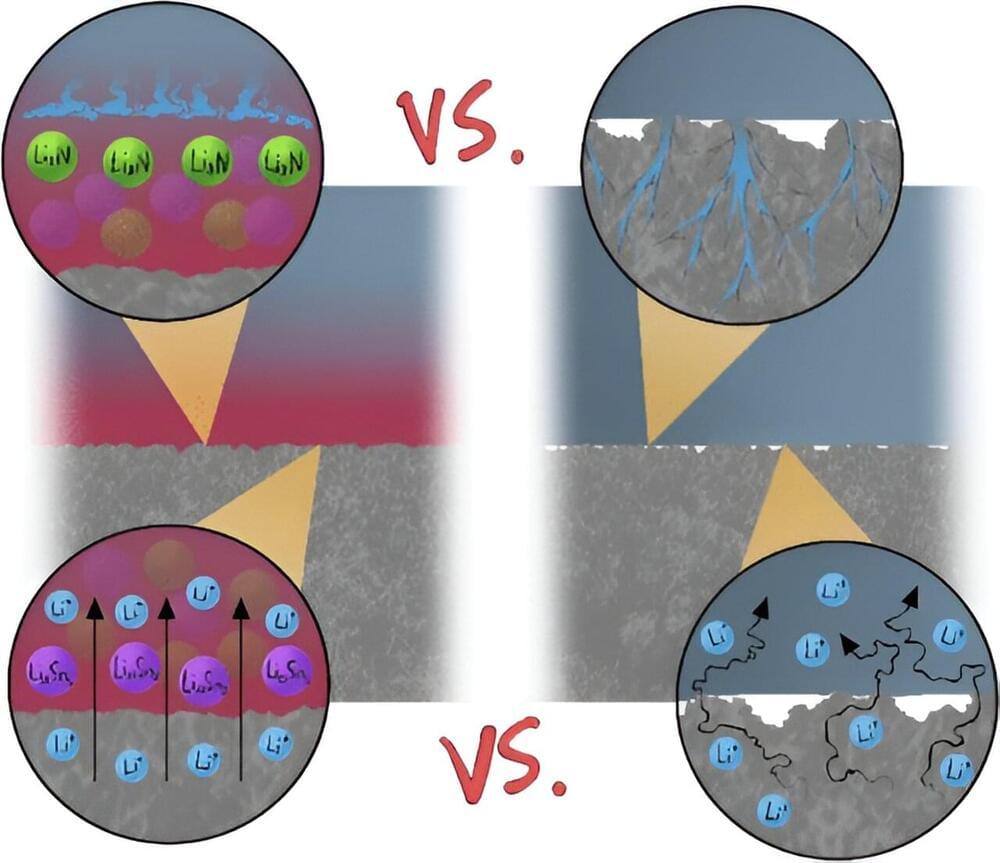
They do have one significant shortcoming, however: they’re prone to short-circuiting. This occurs when a connection forms between the two electrodes inside the cell. A short circuit can result in a sudden loss of voltage or the rapid discharge of high current, both causing the battery to fail. In extreme cases, a short circuit can cause a cell to overheat, start on fire, or even explode.
A leading cause of short circuits are rough, tree-like crystal structures called dendrites that can form on the surface of one of the electrodes. When dendrites grow all the way across the cell and make contact with the other electrode, a short circuit can occur.









Comments are closed.